Fuel Cells
Characteristics
Fuel cells have been around longer than most batteries - the principle of the fuel cell was discovered in 1839 by Sir William Grove. They generate electricity from the reaction of hydrogen with oxygen to form water in a process which is the reverse of electrolysis.
The fuel cell relies on a basic oxidation/reduction reaction, as with a battery, but the reaction takes place on the fuel rather than the electrodes. The fuel cell produces electricity as long as the cell receives a supply of fuel and can dispose of the oxidized old fuel . In a fuel cell, the anode usually is bathed in the fuel; the cathode collects and makes available the oxidant (often atmospheric oxygen). An ion-conducting membrane separates the two, allowing the reaction to take place without affecting the electrodes.
There are six major fuel cell technologies are currently being pursued for different applications each with its own characteristics. Some operate at high temperatures, some use exotic electrode materials or catalysts, all are very complex.
- Alkali
- Phosphoric Acid
- Solid Oxide
- Molten Carbonate
- Proton Exchange Membrane PEM
- Direct Methanol DMFC
They have been proposed for a wide range of applications from powering laptop computers, through automotive traction to high power load levelling.
The most active developments are currently in the automotive sector where the favoured technology is PEM. This promises a high conversion efficiency of over 60% and an energy density of 120 W/Kg. DMFC do not use Hydrogen fuel with its associated supply problems, but the more convenient liquid Methanol. They are less efficient but offer compact and convenient designs suitable for future consumer electronics applications.
How a Fuel Cell Works 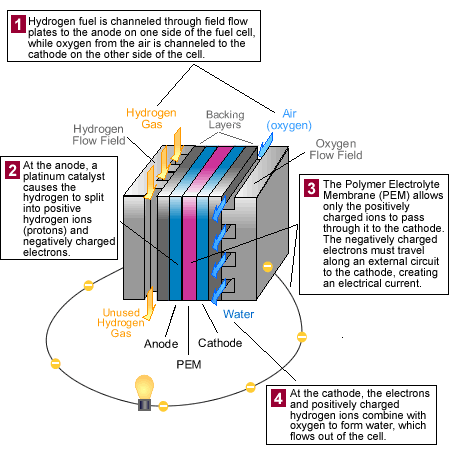
Source: U.S. Department of Energy
The potential power generated by a fuel cell stack depends on the number and size of the individual fuel cells that comprise the stack and the surface area of the PEM.
Advantages
- Fuel cell power is usually proposed as the green, alternative to the internal combustion engine, fuelled only hydrogen and leaving no pollutants other than water.
- Simple fuel requirements needing hydrogen fuel only, taking their oxygen from the air.
- No recharging is necessary.
- No time lost through recharging. (Acts like a perpetual primary cell)
- So long as fuel provided, the cells can provide constant power in remote locations.
- Practical fuel cells already have efficiencies as high as 60%
- Fuel cells deliver maximum efficiency at low power levels.( This is the reverse of the internal combustion engine)
- For transport applications fuel cell vehicles offer higher "well to wheel" (WTW) efficiencies than conventional internal combustion engines.
Well to Wheel Efficiencies

Source: US Department of Energy
CGH2 - Centrally Generated Hydrogen
SGH2 - Station Generated Hydrogen
CIDI - Compression-Ignition Direct-Injection
The graphs show that, compared with an internal combustion engine, the higher "tank to wheel" (TTW) efficiency of the fuel cell power unit more than compensates for the low "well to tank" (WTT) distribution efficiency resulting from the need to compress the hydrogen or to generate it on board.
The fuel cell vehicle however does not offer significant efficiency improvements over the hybrid diesel.
See more on Costs below.
Shortcomings
The environmentally friendly credentials of fuel cells overlook the processes needed to generate and distribute the hydrogen fuel. Fuel cells merely shift the pollution from the vehicle to some other location.
Today, 98% of hydrogen is produced from fossil fuel sources.
According to researchers Andrew and Jim Oswald from Warwick University: To replace petrol and diesel used for road transport in Britain with hydrogen produced by the electrolysis of water would require the building of 100 nuclear power stations or 100,000 wind turbines. If the wind turbines were sited off-shore, this would mean an
approximately 10-kilometre-deep strip of wind turbines encircling the
entire coastline of the British Isles. If sited on-shore then an area
larger than the whole of Wales would have to be given over to wind
turbines.
A major factor inhibiting market take off is the lack of available infrastructure to provide the hydrogen fuel. Hydrogen fuel can be supplied in pure form in cylinders or the on board cylinders can be refilled at special refueling stations. Despite safety precautions there is still a perception by the general public that hydrogen fuel is unsafe.
Alternatively hydrogen can be generated on board, as required, from hydrocarbon fuels such as Ethanol, Methanol, Petrol or Compressed Natural Gas in a process known as reforming. This is not an ideal solution. Reforming generates carbon dioxide as a waste product losing some of the green benefits of fuel cells. It is also expensive and it is like carrying your own chemical plant with you, but it does simplify the fuel supply infrastructure problem, however the fuel could just as easily power an internal combustion engine directly.
Even ignoring these problems there are still many shortcomings in using fuel cells for prime motive power.
The low cell voltage 0.6 - 0.7 Volts means that the system needs a lot of cells to obtain a normal operating voltage of 200 - 300 Volts to power the drive train motor.
Power is generated as required but the process is not reversible within the fuel cell and so, like a primary cell, it can not accept regenerative braking loads. Fuel cells generate electrical energy but they can not store electrical energy.
Fuel cells have a low dynamic range and slow transient response which causes an unacceptable lag in responding to calls for power by the driver. A power boost from a battery or from supercapacitors is therefore needed to achieve the desired system performance.
Most designs need to work at high temperatures in order to achieve reasonable operating efficiencies. To generate the same efficiencies at lower temperatures requires large quantities of expensive catalysts such as platinum.
Low temperature freeze-up of the electrolyte.
Electrodes which are prone to contamination.
Due to the need to use of exotic materials and complex system designs the system are still very expensive.
Theoretically a fuel cell should be all that is needed to power an electric vehicle, however batteries are still needed to support fuel cell systems.
Battery Support
Batteries are needed in fuel cell vehicle applications for the following functions:
- During start- up to heat the fuel cell stack and the electrolyte to the optimum working temperature
- To pump the working fluids through the stack (air, hydrogen, water)
- To power the reformer if hydrogen is generated on board
- To provide short term power boosts to compensate for the fuel cell's slow response to sudden power demands (acceleration)
- To capture regenerative braking energy
- To power the vehicle's low voltage electrical systems
Applications
For automotive applications fuel cells are only suited to hybrid applications for providing the base power load with the demand peaks and troughs, and regenerative braking, being accommodated by batteries or booster capacitors. The fuel cell can therefore be dimensioned to work at its optimum working point, providing the average power rather than the peak power requirement permitting significant cost savings.
Fuel cells have been used successfully in aerospace applications.
Simple low power demonstrator kits are available for education purposes.
Perhaps the best applications for fuel cells will be for high power load levelling.
Prototypes of Direct Methanol cells are currently being trialled for mobile phone and laptop computer applications.
Costs
For a true comparison of alternative system efficiencies, costs and benefits, each alternative should be based on the same fuel source. Using oil as the original source of the energy the "well to wheel" cost provides a rational comparison of the energy utilisation efficiency of different systems.
But oil is not the only source of energy. Electrical energy used to power electrical vehicles or to produce the hydrogen to feed the fuel cells can be derived from a wide variety of sources. These may include power stations fuelled by oil, or coal or hydro or nuclear power, or renewable resources such as wind, wave and solar power. There can thus be a huge variation in costs and environmental impacts depending on the methods used to supply the necessary fuel.
Although many working systems for different applications have been built, practical, cost effective products are still perhaps ten years away.
|

![]() Print This Page || Home || FAQ || Site Map || Legal || Privacy Promise || Contacts
Print This Page || Home || FAQ || Site Map || Legal || Privacy Promise || Contacts

