Battery and Energy Technologies |
 |
|
Direct Conversion of Heat Energy to Electrical Energy (1)
Thermocouple Electric Generators
When any electrical conductor is subjected to a thermal gradient, by heating one end while maintaining the other end at a low temperature, it will generate a voltage between the hot and cold ends. This is known as the Thomson effect and is principle used for the direct conversion of heat energy into electrical energy.
ThermocouplesThe phenomenon of thermoelectricity was first observed in 1821 by by the German physicist Thomas Johann Seebeck who noticed that when a loop was made from wires using two dissimilar metals, a voltage appeared between the junctions of the wires if one junction was hotter than the other. Such a loop made with dissimilar metals became known as a thermocouple and the phenomenon was named the Seebeck effect in his honour. The voltage generated by the thermocouple is very small and many thermocouples are required to make a practical thermoelectric generator.
Semiconductor Thermocouples For over a century thermocouples were made from metallic conductors and though many different metals were investigated, efficiencies rarely exceeded 3%. With the advent of semiconductors the efficiency of thermoelectric generators was greatly increased and by the 1950's, generator efficiencies had reached 5% and Peltier cooling from ambient to below 0°C was achieved. See the section on Semiconductors for an explanation of how thermocouples work.
Thermocouple Performance
The Seebeck Coefficient Kelvin showed that for small temperature differences the voltage produced between the hot and cold ends of a single conducting rod is proportional to the temperature difference between the two ends. The proportionality constant S is now known as the Seebeck coefficient and is defined as: S = Δ V / Δ T where Δ T is the temperature difference between the two ends of a material and Δ V is the thermoelectric voltage generated. So that the voltage generated is given by: Δ V = S*Δ T It is thus a measure of the magnitude of an induced thermoelectric voltage in response to a temperature difference across the material. For most conductors the voltage created is tiny, just a few microvolts per degree difference in temperature. For semiconductor materials the coefficient may be between 100μV/°K and 300μV/°K, in any case still very small. This is mainly because the kinetic energy of the charge carriers in semiconductors is strongly temperature-dependent, whereas in metals it is not so strongly temperature-dependent. More generally the Seebeck coefficient is non-linear, and depends on the material of the conductor, its molecular structure and the absolute temperature. The Seebeck voltage does not depend on the temperature distribution along the conductor but only on the temperature difference between the ends. The Seebeck coefficient is often incorrectly referred to as the thermoelectric power or thermopower (it is a voltage not a power).
Thermoelectric Materials Ideal thermoelectric materials should have the following properties:
For comparison purposes the usefulness of thermoelectric materials for electricity generation as well as for heating and cooling can be characterised using a figure of merit incorporating these properties. The figure of merit Z of a thermoelectric material is a measure of its efficiency as an energy conversion component and is defined as : Z = σS2 λ Materials with high thermoelectric figures of merit are typically heavily doped semiconductors and for many years the best materials had a figure of merit of around 1. Recent advances in materials science have increased this number to around 4.
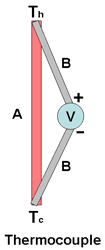
Thermocouple Output Voltage In practice, to extract useful current from the ends of a conducting rod requires the connection of wires to the end points, essentially forming a second conductor in parallel between the heat source and the heat sink. The voltage generated in the wires will thus oppose the voltage generated across the rod and the net voltage generated will be the difference between the voltages generated across the rod and across the wires. The circuit arrangement containing two dissimilar metals forms a thermocouple.
The thermoelectric voltage generated in a single conductor is already very small. The connection of wires across the conductor to extract the electrical energy introduces an opposing voltage in the circuit so that the net useful voltage available is even smaller.
The diagram below illustrates the voltage developed by the thermocouple.
This is the physical basis for a thermocouple, which is used often for temperature measurement and in special circumstances for power generation. See Practical Devices below
Thermoelectric Efficiency The efficiency of a thermocouple depends on the fundamental properties of the thermoelectric materials used in its construction and the only way to improve it is to develop new materials with a higher figure of merit. Despite 180 years of experimenting with a myriad of different materials, typical thermoelectric conversion efficiencies are still only around 3% and efficiencies above 10% have never been achieved. The best efficiencies achieved to date in spacecraft applications are around 7% to 8%, similar to amorphous Silicon (Si) solar cells, but inferior to the 24% achieved by solar cells using exotic materials.
There are much more efficient ways of turning heat into electricity than by using thermoelectric devices.
Practical Thermoelectric DevicesBecause conversion efficiencies are very low, thermocouple applications are limited mostly to low power devices. Costs are also very high which further limits their potential uses.
Higher electrical outputs can be achieved in Seebeck applications at the expense of using more heat by increasing the temperature difference between the hot and cold surfaces. The limiting factors here are the thermal and chemical stability of the thermoelectric material at high temperatures and the ability to remove the surplus heat from the cold surface. Similarly the cooling performance of Peltier devices can be improved by using higher currents but the same limiting factors apply, except that in this case, the surplus heat must be removed from the hot surface.
Typical thermocouple applications using the Seebeck effect are temperature measurement, heat sensing and the detection of radiation in bolometers. Thermoelectric batteries powered by body heat are also used in portable medical monitoring devices.
Since the energy available from a single thermocouple is very small, arrays of thermocouples must be used to construct thermoelectric devices capable of handing practical amounts of power. Higher power devices can be made by connecting thermocouples in series to increase the voltage capability and in parallel to increase the current capacity. Such an array of thermocouples is called a thermopile. Thermopile
Thermoelectric generators can be used in much the same way as photovoltaic devices and the same electrical ancillary circuits can be used. For example higher voltage outputs can be achieved by using the array to drive a DC/DC converter.
TEGs have been used for some time in Radioisotope Thermoelectric Generators (RTGs) to provide portable power in spacecraft applications using the heat from the decay of radioactive isotopes such as Plutonium 238. See Nuclear Batteries .
More recently the possibility of using thermocouple arrays in automotive applications to recover waste heat from engine exhaust gases is being investigated. With an exhaust gas temperature of 250°C and a coolant temperature of 50°C, power outputs of over 300 Watts have been achieved but this drops to 150 Watts when the coolant temperature increases to 90° C
See also Direct Conversion of Heat Energy to Electrical Energy (2) the AMTEC Thermal Battery
Return to Electrical Energy Overview
|
||||||||||||||||||||||||||||||
![]() Print This Page || Home || FAQ || Site Map || Legal || Privacy Promise || Contacts
Print This Page || Home || FAQ || Site Map || Legal || Privacy Promise || Contacts
Woodbank Communications Ltd, South Crescent Road, Chester, CH4 7AU, (United Kingdom)
Copyright © Woodbank Communications Ltd 2005